ULTOMIRIS® Was Proven to Provide
Continuous Control Over gMG Symptoms
After the first 26 weeks of a clinical trial, people on ULTOMIRIS saw:

More than 2x greater improvement in activities of daily living such as:
Seeing
Chewing
Breathing
Brushing teeth
Combing hair
Rising from a chair

More than 3x greater reduction in muscle weakness, improving physical functions such as:
Eye and facial movements
Swallowing
Speaking
Hand gripping
Head lifting
Limb stretching
*Versus placebo from baseline to Week 26 of the clinical trial, according to the Myasthenia Gravis Activities of Daily Living (MG-ADL) scale. In the study, the baseline MG-ADL total score for the 86 people on ULTOMIRIS was 9.1; for the 89 people on placebo, it was 8.9. Many people continued taking other medicines throughout the study.
†Versus placebo from baseline to Week 26 of the clinical trial, according to the Quantitative Myasthenia Gravis (QMG) scale. In the study, the average baseline QMG total score for the 86 people on ULTOMIRIS was 14.8; for the 89 people on placebo, it was 14.5. At Week 26, the average change in score from baseline was -2.8 for people receiving ULTOMIRIS and -0.8 for those receiving placebo. Many people continued taking other medicines throughout the study.
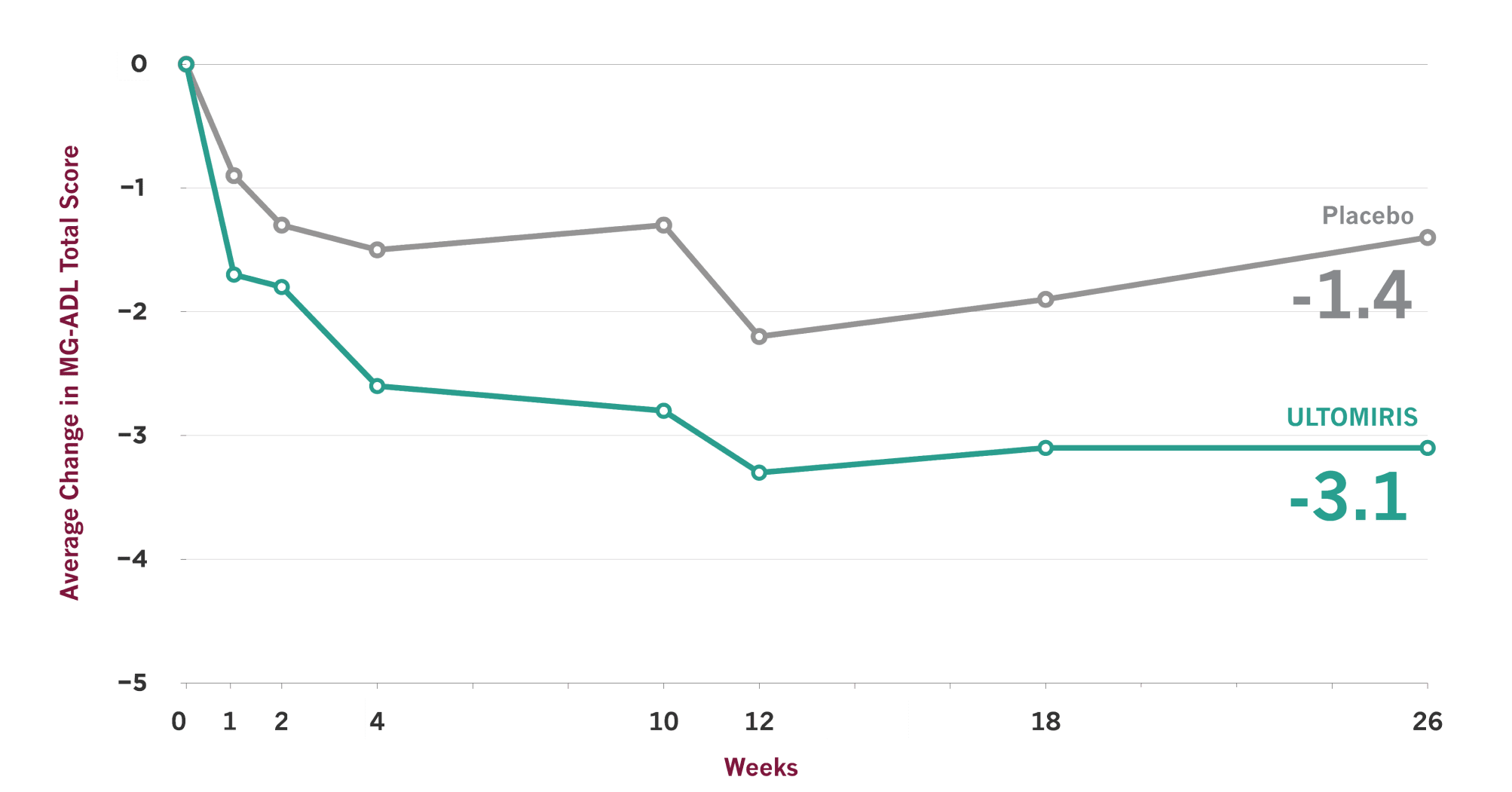
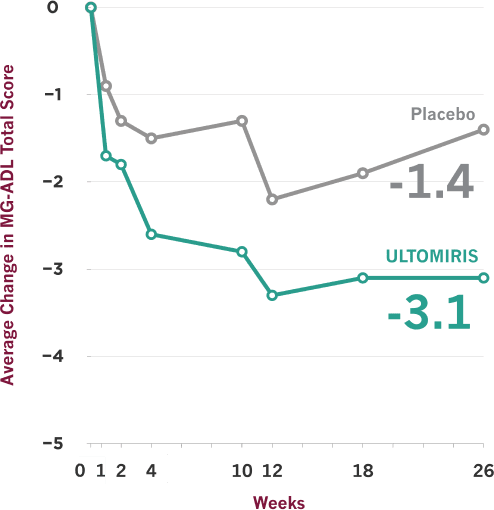
ULTOMIRIS demonstrated effectiveness vs placebo at Week 26, and some people showed earlier improvement
See the results
Study Details and Limitations
- On the scale used to measure these results, lower scores indicate improved gMG symptoms
- Clinical trials are designed to make sure results are meaningful and not due to chance. Comparisons between ULTOMIRIS and placebo at any time point before Week 26 were part of the planned study of ULTOMIRIS, but the primary goal of the study was to measure them at Week 26, so no conclusions should be drawn from the data prior to Week 26
- Placebo is an inactive substance or treatment that looks the same and is given the same way as the medication being studied
- Individual results may vary. Not everyone who takes ULTOMIRIS will experience the same results
How were these results measured?
The Myasthenia Gravis Activities of Daily Living (MG-ADL) scale is a patient-reported symptom improvement questionnaire that was used in the ULTOMIRIS study to measure the impact of gMG symptoms on 8 key daily activities and functions. MG-ADL total scores range from 0 to 24, with higher scores indicating more severe gMG symptoms.
Another questionnaire called the Quantitative Myasthenia Gravis (QMG) scale was also used in the ULTOMIRIS study. The QMG scale is a 13-item doctor-reported symptom improvement scale that assesses muscle weakness. QMG total scores can range from 0 to 39, with higher scores indicating more severe gMG symptoms.
ULTOMIRIS was studied in a 26-week clinical trial that included adults with varying degrees of severity of anti-AChR antibody-positive gMG
The CHAMPION-MG trial measured the impact of ULTOMIRIS (ravulizumab-cwvz) on daily activities and muscle weakness. It included 175 people who were randomly split into 2 groups: those taking ULTOMIRIS (86 people) and those receiving placebo (89 people)
- The MG-ADL scale measured the effects of gMG symptoms on activities of daily living and physical functions
- The QMG scale measured muscle weakness
- Over 90% of people in the trial had mild or moderate gMG‡
- At their first dose of ULTOMIRIS, most people were taking an immunosuppressive therapy. If people were receiving immunosuppressive therapies at the start of the study, they were required to continue taking them at stable doses throughout the initial study period of 26 weeks
- After Week 26 of the trial, all study participants were eligible to receive ULTOMIRIS for an extension period of up to 4 additional years
‡As defined by Myasthenia Gravis Foundation of America (MGFA) clinical classification.
Additional Data for ULTOMIRIS
Medicines are studied in clinical trials to find out if they can help people effectively and safely
Clinical trials—like the one in which ULTOMIRIS was studied—have a few specific goals that are decided before they begin.
- The results below were not preplanned study goals, so no conclusions can be drawn. Talk with your healthcare team about any questions you may have
Alexion is committed to the continued analysis of trial data to better understand the treatment of gMG.
People who started ULTOMIRIS within 2 years of gMG diagnosis experienced 4.3 points of improvement in MG-ADL total score at Week 26, vs 2.9 points for people who started ULTOMIRIS more than 2 years after diagnosis.
Of 175 people in the study, 35 started the study within 2 years of gMG diagnosis (19 received ULTOMIRIS) and 140 started more than 2 years after diagnosis (67 received ULTOMIRIS).
82% of people experienced at least 3 points of improvement with ULTOMIRIS by Week 60 of the clinical trial.
At Week 60 (34 weeks after people who received placebo in the initial 26 weeks of the clinical trial had switched to ULTOMIRIS, and after those receiving ULTOMIRIS had continued to take it for an additional 34 weeks), their data up to that point were collected for analysis. Of the 139 people who stayed in the study until that time point, 82% had at least 3 points of improvement in MG-ADL total score. The extension period was designed to measure safety, so no conclusions should be drawn.
Individual results may vary. Not everyone who takes ULTOMIRIS will experience the same results.
I did notice a change with ULTOMIRIS. I notice that I don't slur my words as long. I don't choke as much.
Mike
Real ULTOMIRIS Patient
Mike, living with gMG, has received compensation from Alexion Pharmaceuticals, Inc.
Mike has a family member who is employed by Alexion, including at the time Mike started treatment.
Download this helpful discussion guide full of information and ideas for talking to your doctor about ULTOMIRIS
Download The Doctor Discussion GuideWant to learn more about how ULTOMIRIS may help?
Call us at 1-877-GMG-ULTO (877-464-8586)
Receive information and updates

Join our gMG community

